インフォメーション
LICENSE厚生労働大臣許可医療機関
第二種・第三種再生医療等提供計画 承認済
リペアセルクリニックは、第二種・第三種再生医療提供計画を厚生労働省に提出し受理されました。
-
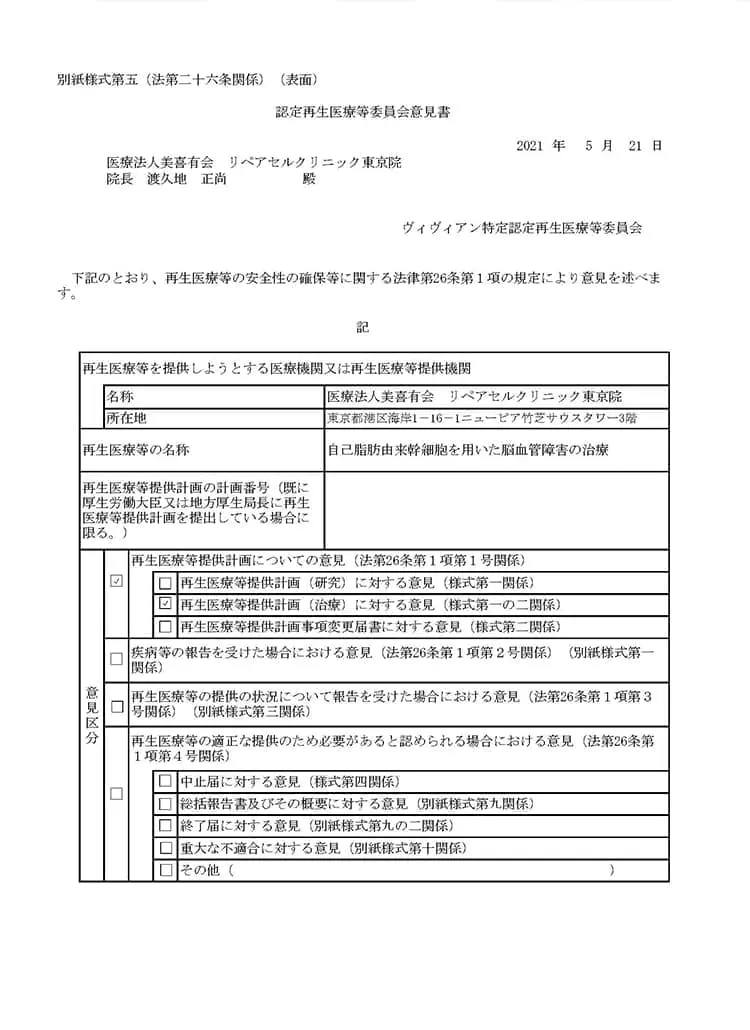
自己脂肪由来幹細胞を用いた脳血管障害の治療
-
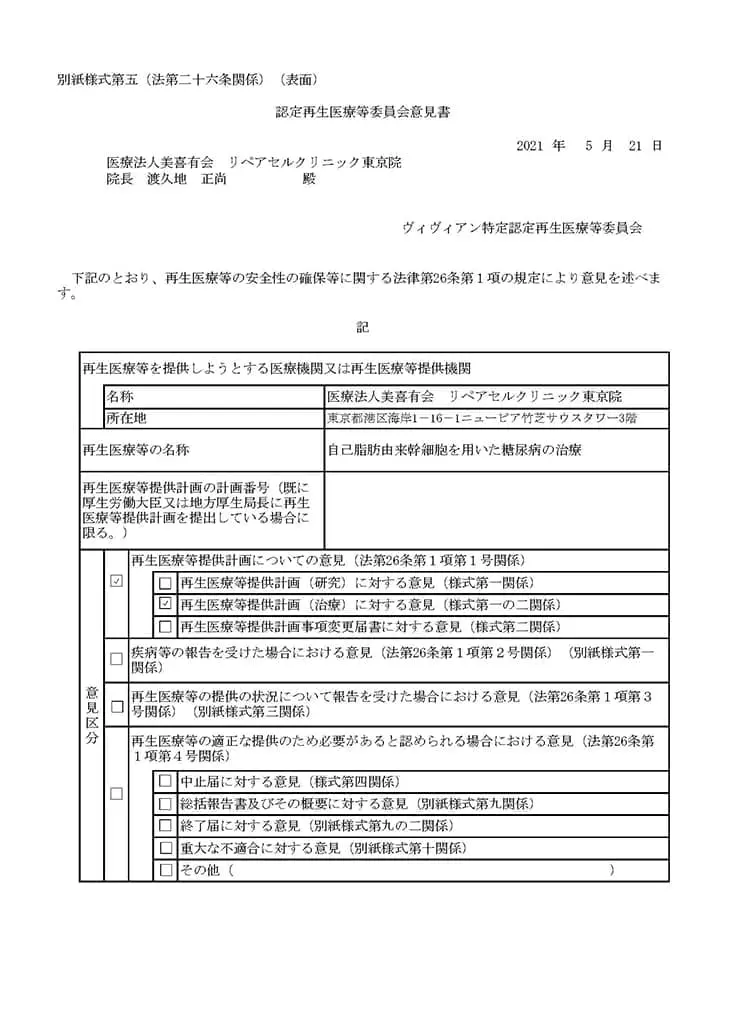
自己脂肪由来幹細胞を用いた糖尿病の治療
-
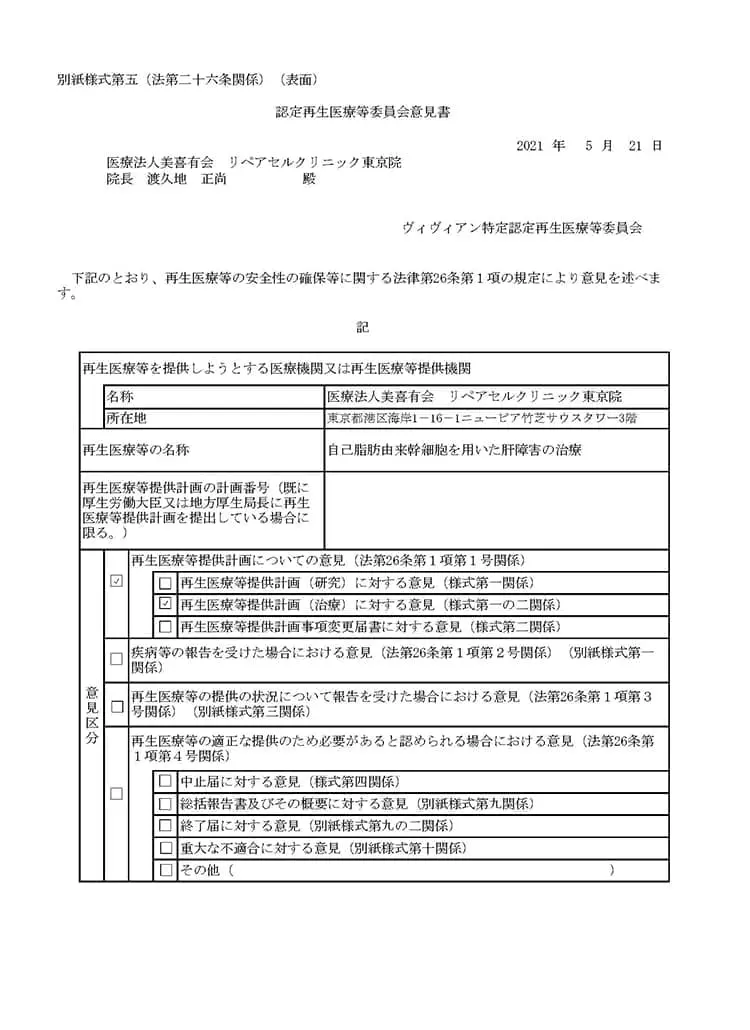
自己脂肪由来幹細胞を用いた肝障害の治療
-
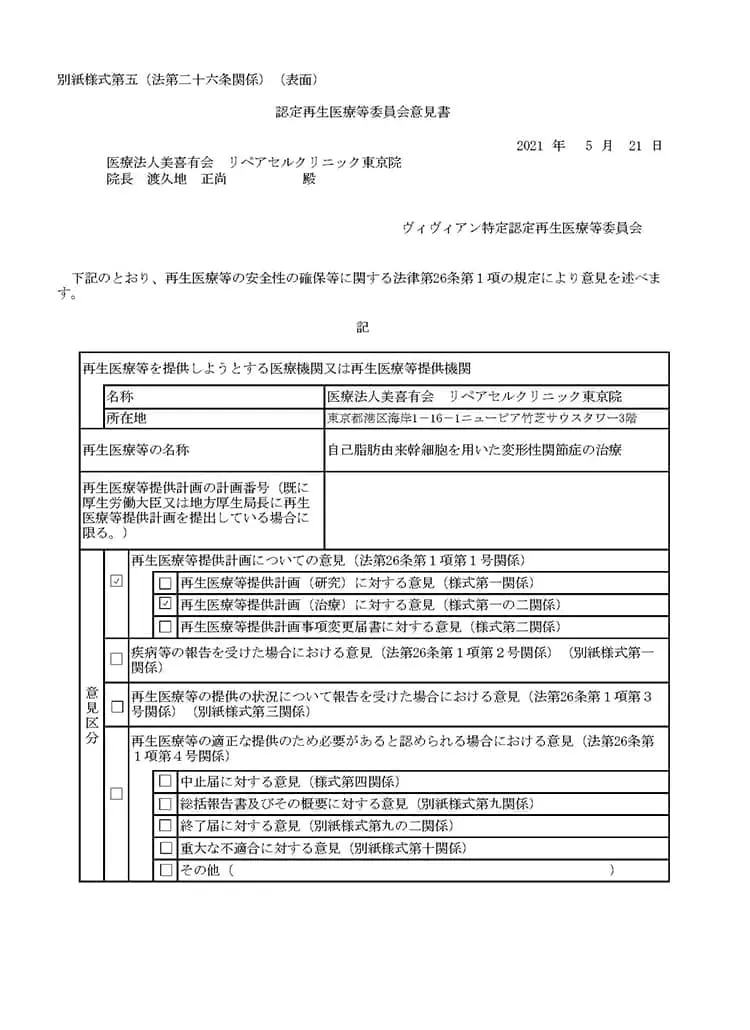
自己脂肪由来幹細胞を用いた変形性関節症治療
-
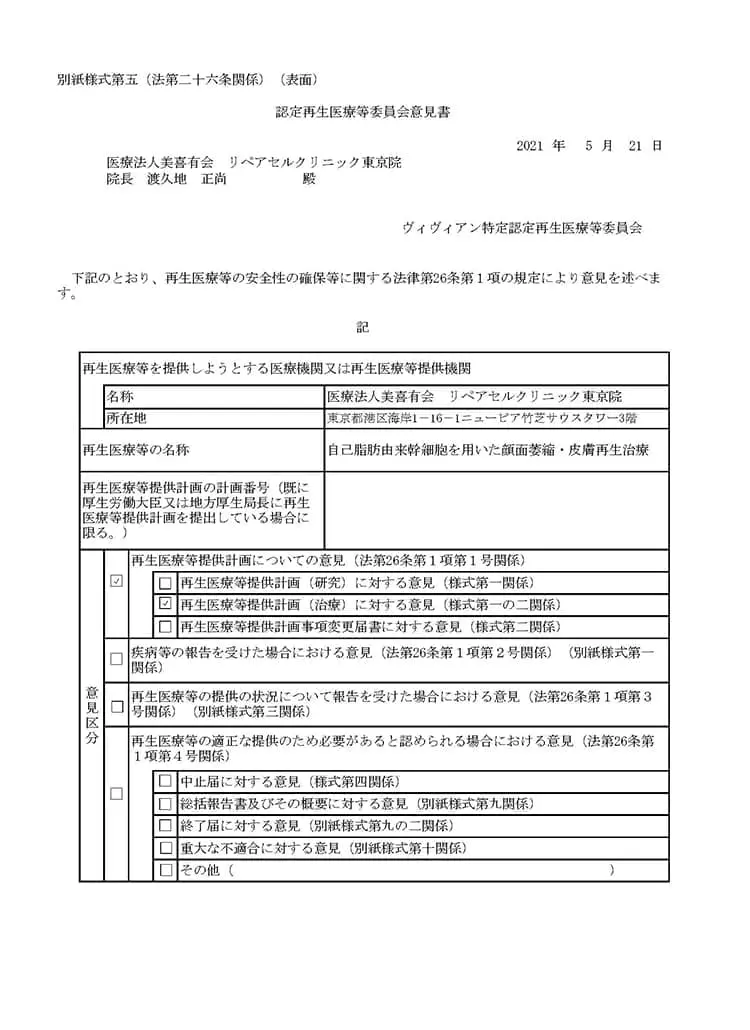
自己脂肪由来幹細胞を用いた顔面萎縮症、皮膚再生治療
-
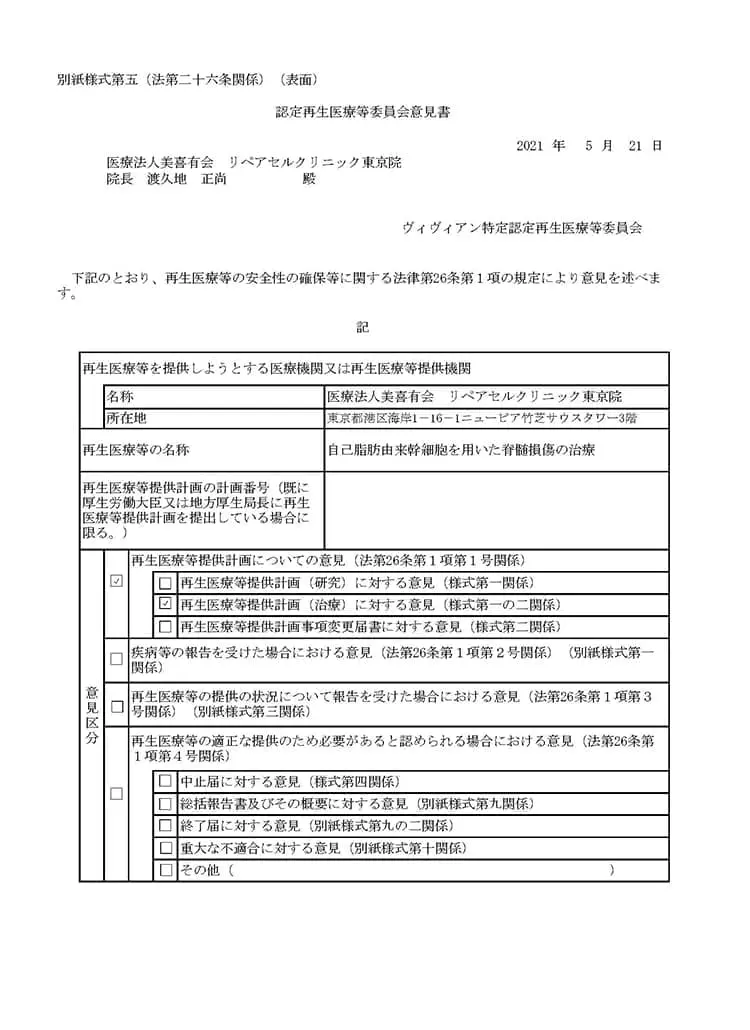
自己脂肪由来幹細胞を用いた脊髄損傷の治療
-
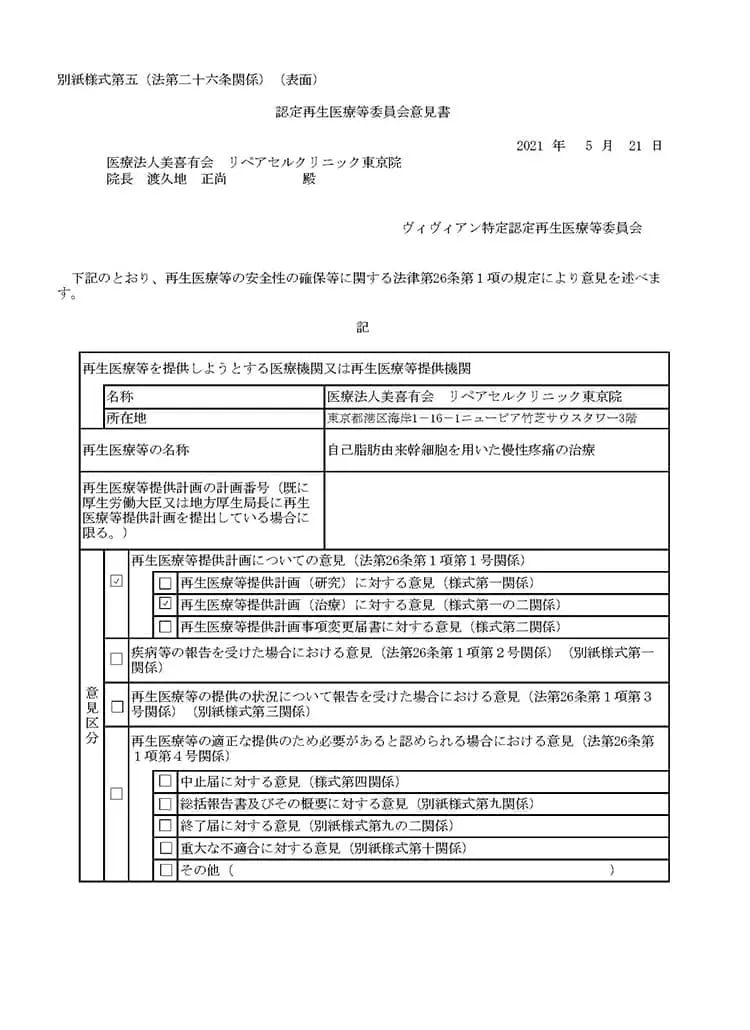
自己脂肪由来幹細胞を用いた慢性疼痛の治療
-

多血小板血漿(PRP)を用いた変形性関節症の治療
-
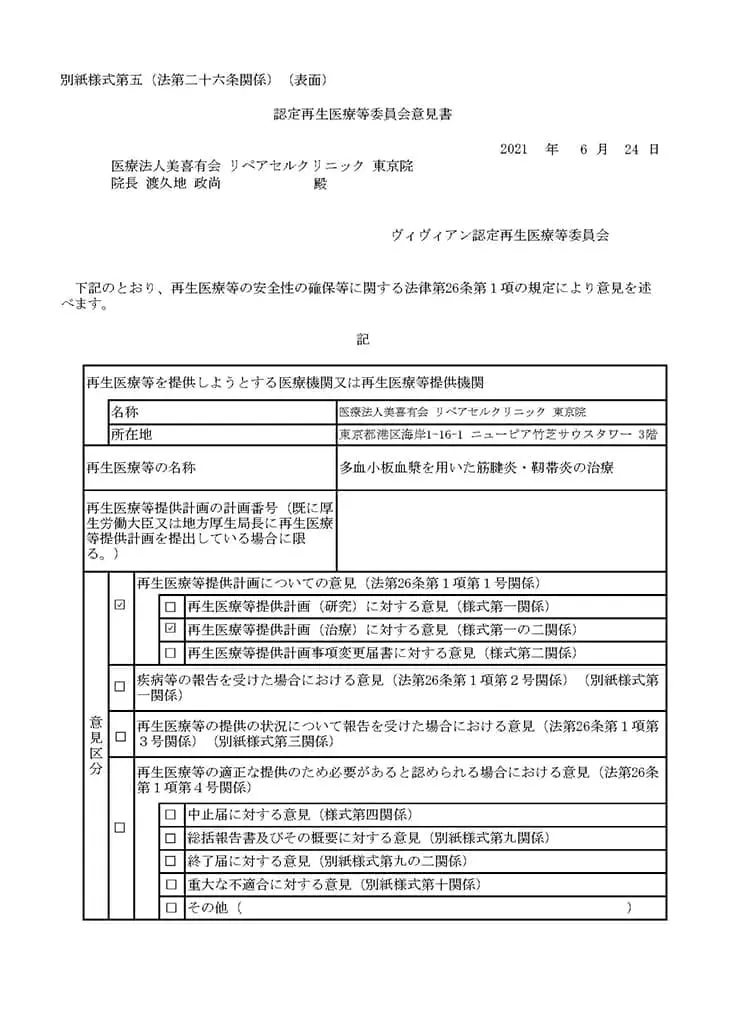
多血小板血漿(PRP)を用いた筋腱炎、靭帯炎の治療
-
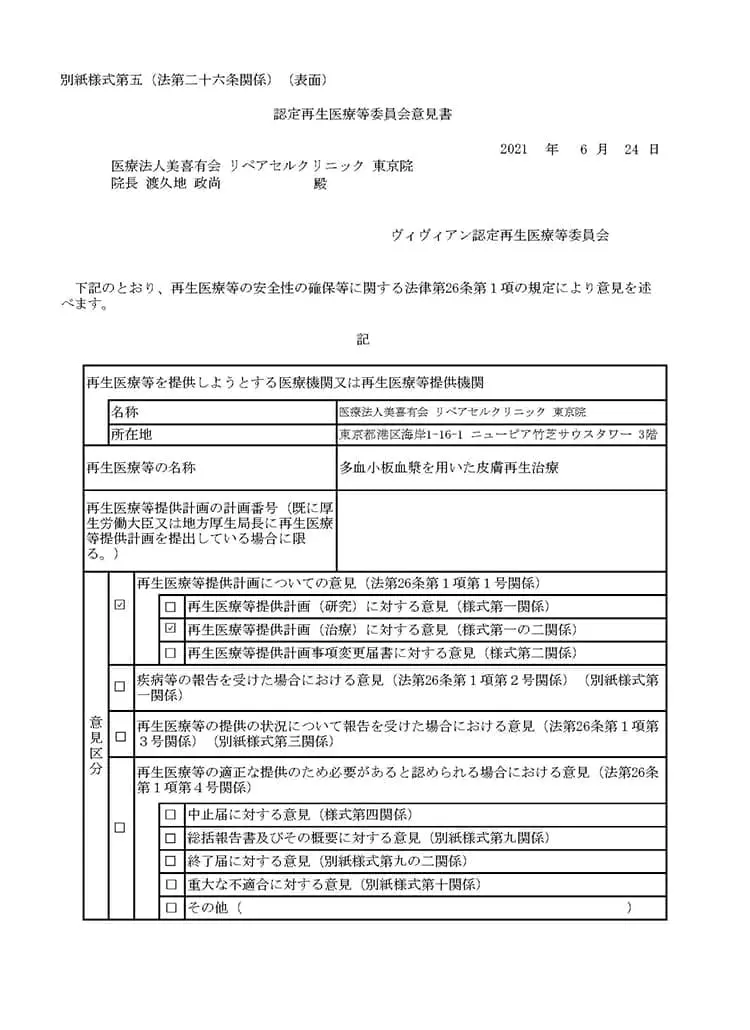
多血小板血漿(PRP)を用いた皮膚再生療法

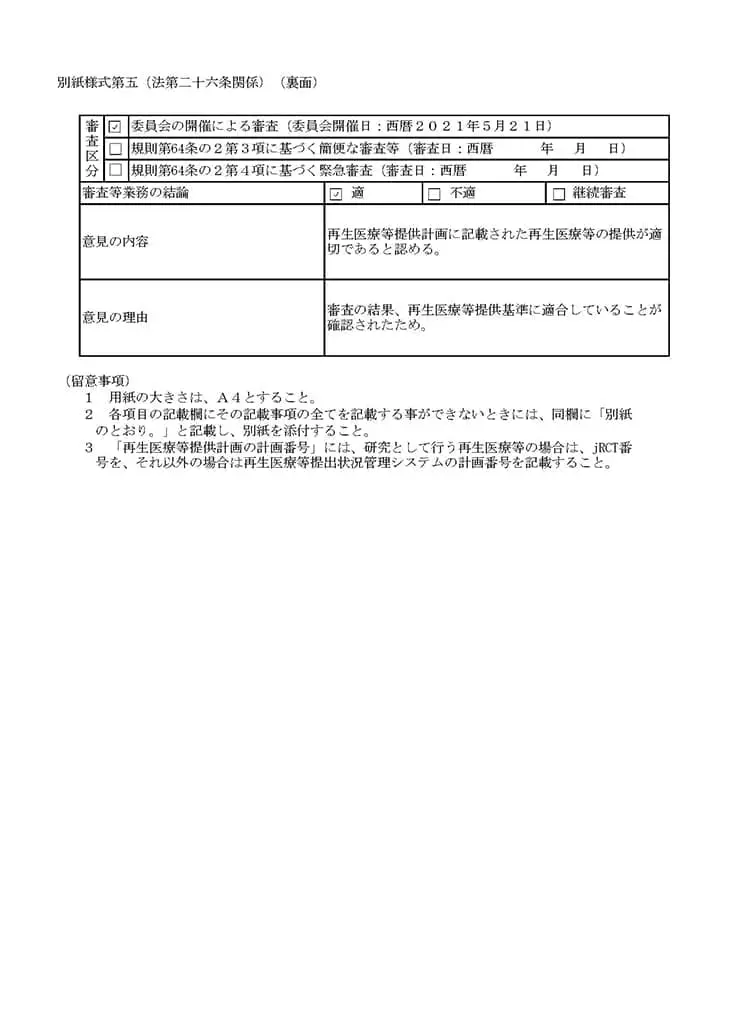

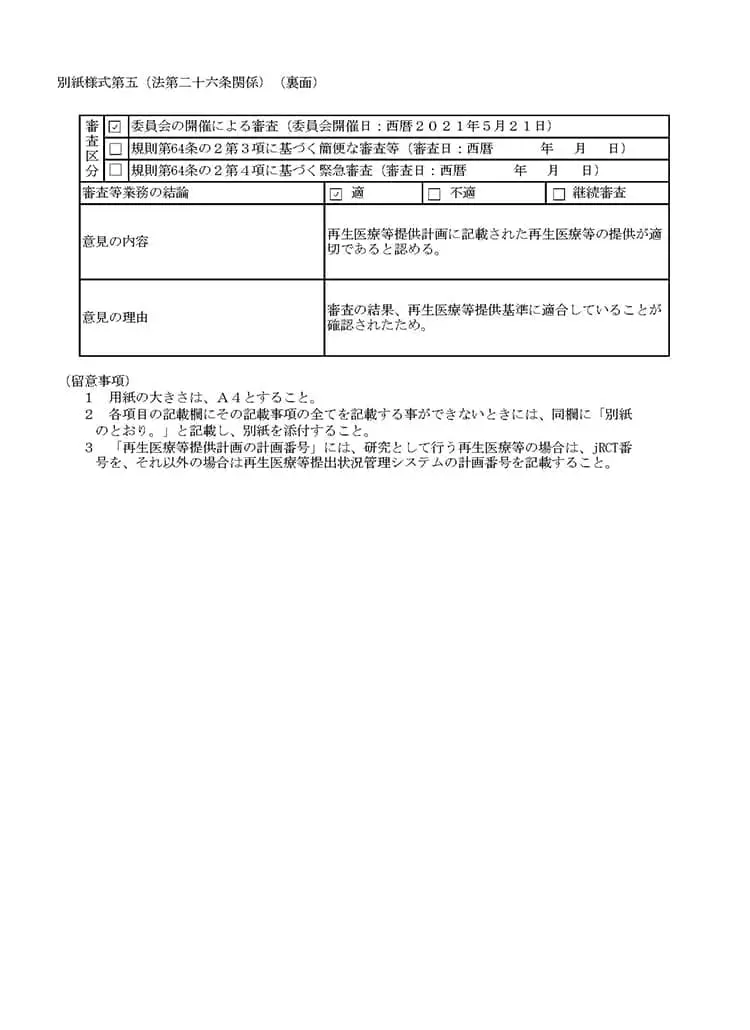





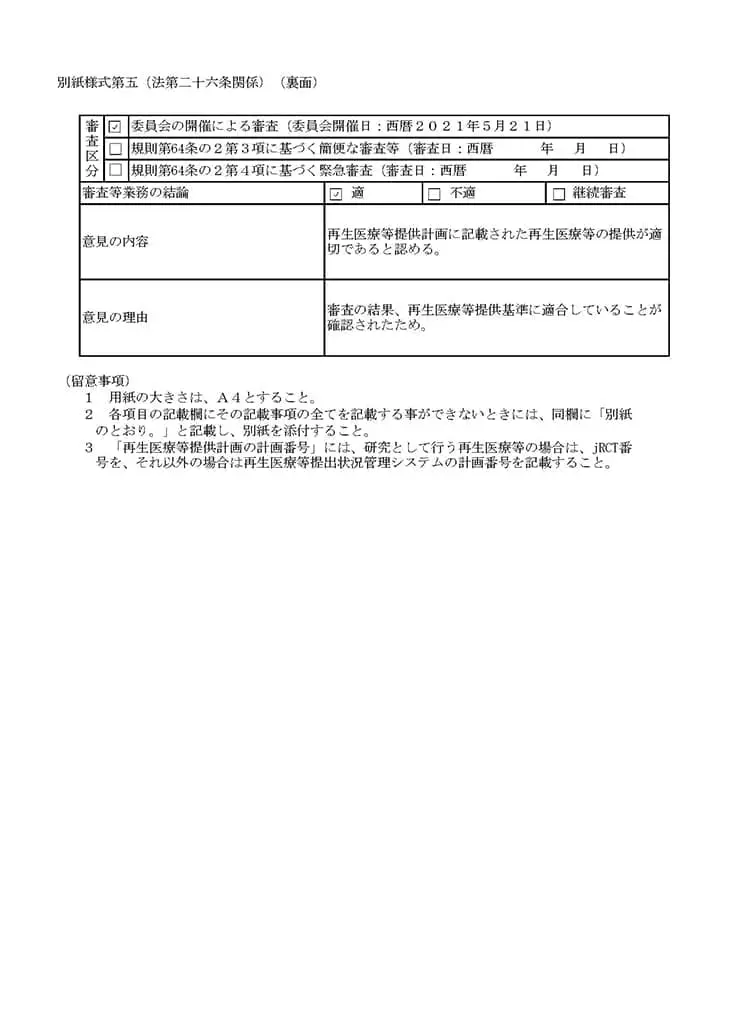

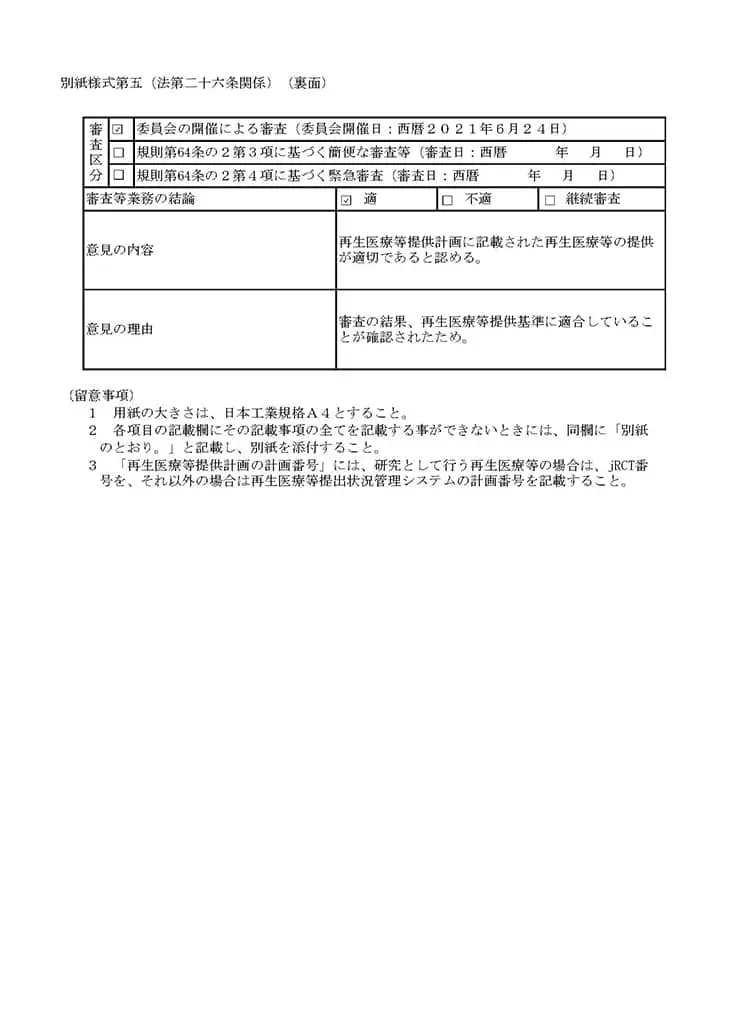


当クリニックでは、国内では数少ない自己の幹細胞を用いた「変形性関節症」「脳卒中」「糖尿病」「肝障害」「肌の再生」などの最先端の再生医療および、PRP(多血小板血漿)の関節内投与を再生医療安全確保法のもと、自由診療にて提供しています。再生医療とは、厚生労働省が認めた特定認定再生医療等委員会において、厳しく審査が行われ、治療の妥当性・安全性・医師体制などが適切と認められる事ではじめて厚生労働省に治療計画を提出することができます。
自分の細胞を活用し、
蘇らせる「再生医療」とは?
薬での治療は限界ではないだろうか。本当に手術は必要だろうか。
そんな思いで悩んだり、あきらめたりしていませんか?
ケガをしても傷跡が少しずつ薄くなる・・
当たり前のようですが、あなた自身の細胞には、弱ったところ、傷ついたところを修復するチカラがあります。
その細胞のチカラを最大限に引き出して治療を行うことを「再生医療」と呼び、おすすめしています。
リペアセルクリニックの特長
当クリニックは、疾患・免疫・美容という分野すべてを、自己細胞を用いた最先端の医療で行うことができる国内でも珍しい部類の医療機関です。
CPC(細胞加工施設)の高い技術により、冷凍しない方法で幹細胞を投与できるので高い生存率を実現。
ご自身の細胞や血液を利用するため、アレルギーや拒絶反応といった副作用の心配が少ないおすすめの治療方法です。
- 1億の細胞を
投与可能※但し適応による - 高い
安全性 - 入院不要
日帰り - 身体への
負担が少ない - 高い技術力を
もったCPC

できなくなったことを、再びできるように
わたしは医学部卒業後、大阪市立大学(現大阪公立大学)医学部附属病院で関節や脊椎の疾患、脊髄損傷などの外来や手術に従事しました。また、救命救急センターで脳卒中や心疾患などの内科疾患を幅広く経験しました。
膝や股関節の人工関節については専門病院で約1000例以上の手術とリハビリを手掛け、その後生まれ育った地元でクリニックを開業いたしました。開業してからも患者さまの痛みや苦痛を少しでも和らげ、人生をより楽しく、明るい気持ちで過ごしてもらいたいという思いを持ち続けて診療を行い、地域の皆さまに愛される医療を目指し在宅医療や訪問看護にも力を注いで参りました。
そんな道を進みながらも、わたしは常に患者さまにどうやったらより質の良い医療を提供できるだろう、という思いを抱き続けて模索を繰り返していました。やはり保険診療の範囲では限界があり、患者さまの生活の質を一定以上回復させることは叶わないからです。
そんな時に再生医療と出会い、新たな可能性を見出すことができました。
再生医療の凄いところは、安全性を担保しながら治療を行う事が出来、しかも一般的には治療が難しいと言われる症状に対しても劇的な回復を見込めるという事です。脳血管疾患や脊髄損傷などで深刻な後遺症を持つ方がみるみる回復していく様に、わたし自身も驚きの連続でした。
また、膝、股関節、肩など関節疾患により大好きなスポーツが出来なくなった方が復帰されたり、手術をするしか選択肢がなかった方に、手術に代わる新たな選択肢をご提供する事が出来るようになった事で、多くの患者さまから「この治療を受けて本当によかった」と大変喜ばれております。
「これ以上回復させることはできないのが常識」という概念を覆したのですから、本当に凄いことです。もちろん再生医療は不治の病を全て治すことのできる万全な治療ではありません。しかし、一般的な医療の効果をはるかに超えて結果を出せる可能性がある事を、わたしはたくさんの実績を通して確信に変えています。
そしてもちろん、脳血管疾患や脊髄損傷、整形外科分野の疾患においてリハビリも欠かせない大事な要素の一つです。再生医療×リハビリの相乗効果で回復の幅がかなり広がることでしょう。
再生医療は、わたしが求め続けていた「人生をより楽しく、明るく過ごしてもらいたい」という医療に対する思いそのものだと感じました。現在も日々たくさんの方々にご来院いただき、たくさんの笑顔を見ることが叶っています。患者ご本人さまやご家族が泣いて喜ぶ瞬間は、何度立ち会っても感慨深く、私の原動力になっております。

再生医療における無限の可能性
再生医療の治療は、幹細胞という細胞を用いて行う治療です。幹細胞はとても小さく、身体の中をすみずみまで巡り、人の手の及ばない細かな箇所にもアプローチしてくれるものですので、この治療はまだまだ無限の可能性を秘めており、今後も様々な症例が報告されることでしょう。様々な疾患で苦しむ方の希望になるだろうと、わたしは思っています。
また、当院では患者さまご自身の細胞を使いますので安全に治療をお受けいただけます。どうか安心してご来院ください。
「できなくなったことを再びできるように」
人生100年時代、皆様がより楽しく毎日を過ごせることのお手伝いができれば幸甚の至りでございます。
医療法人美喜有会理事長 坂本 貞範
Sadanori Sakamoto
略歴
- 1997年3月
- 関西医科大学 医学部卒
- 1997年4月
- 医師免許取得
- 1997年4月
- 大阪市立大学(現大阪公立大学)医学部附属病院 勤務
- 1998年5月
- 大阪社会医療センター附属病院 勤務
- 1998年9月
- 大阪府立中河内救命救急センター 勤務
- 1999年2月
- 国立大阪南病院 勤務
- 2000年3月
- 野上病院 勤務
- 2003年3月
- 大野記念病院 勤務
- 2005年5月
- さかもとクリニック 開設(2023年3月譲渡)
- 2006年12月
- 医療法人美喜有会設立 理事長就任
- 2019年6月
- リペアセルクリニック大阪院 開設
- 2021年5月
- リペアセルクリニック東京院 開設
- 2023年12月
- リペアセルクリニック札幌院 開設
主な医学論文•学会発表
- 論文名:透析患者に対する鏡視下手根管開放術の費用と手術手技
論文掲載:日本透析医学会雑誌(1340-3451)37巻Suppl.1 Page770(2004.05) - 論文名:IBBC手技を使用したTKAの中期成績
論文掲載:日本人工関節学会誌(1345-7608)30巻 Page35-36(2000.12) - 論文名:寛骨臼巨大骨欠損の再置換法とその成績 同種骨による欠損壁の修復と水酸アパタイト顆粒による空洞の修復
論文掲載:日本整形外科学会雑誌(0021-5325)74巻2号 Page S319(2000.02) - 論文名:骨セメントと骨界面に水酸アパタイト顆粒を介在させる界面バイオアクティブ骨セメント手技(IBBC)
論文掲載:日本整形外科学会雑誌(0021-5325)74巻3号 Page S666(2000.03) - 論文名:外反母趾手術chevron法に対するPLAの使用経験
論文掲載:中部日本整形外科災害外科学会雑誌(0008-9443)42巻1号 Page241-242(1999.01) - 論文名:亜急性に経過した膝蓋骨骨髄炎の1例
論文掲載:中部日本整形外科災害外科学会雑誌(0008-9443)41巻4号 Page1107(1998.07)
当院で再生医療を
サポートする専門医

藤間 保晶 院長
Yasuaki Toma
略歴
- 1995年3月
- 三重大学医学部学科卒業
- 1995年4月
- 奈良県立医科大学 整形外科入局
- 1996年1月
- 奈良県立奈良病院 救命救急センターおよび麻酔科(救急医療、麻酔)
- 1997年1月
- 三重県大台厚生病院 整形外科(へき地医療)
- 1998年1月
- 大阪松原市立病院 整形外科
- 2000年1月
- 済生会富田林病院 整形外科
- 2002年1月
- 奈良県立三室病院 整形外科
- 2004年7月
- 済生会奈良病院 整形外科
- 2006年7月
- 市立奈良病院 整形外科
- 2008年7月
- 国立病院機構奈良医療センター 整形外科・リハビリテーション科
- 2017年1月
- 市立奈良病院 整形外科・リハビリテーション科
- 2019年4月
- 石心会(新東京石心会)グループ さいわい鶴見病院 関節外科
主な医学論文•学会発表
-
論文名:Early bone in-growth ability of alumina ceramic implants loaded with tissue-engineered bone再生培養骨を導入したアルミナセラミックス製インプラントの早期骨形成能について
著者:Tohma Y, Tanaka Y, Ohgushi H, et al.
論文掲載:Journal of Orthopaedic Research 24(4): 595-603, 2006. -
論文名:Bone marrow-derived mesenchymal cells can rescue osteogenic capacity of devitalized autologous bone自家培養骨髄由来間葉系細胞による壊死骨の骨形成能救済技術の開発
著者:Tohma Y, Ohgushi H, Morishita T, et al.
論文掲載:Journal of Tissue Engineering and Regenerative Medicine 2(1): 61-68, 2008. -
論文名:Basic and Clinical Research into Alumina Ceramic Artificial Joint Prosthesis loaded with Tissue-engineered Bone再生培養骨を導入したアルミナセラミックス製インプラントを用いた基礎研究および実際の人工足関節手術での治療成績
著者:Tohma Y, Tanaka Y, Ohgushi H, et al.
論文掲載:Tissue Engineering Research Trends, Nova Science Publishers, Inc.: 183-197, 2008.
-
4. 論文名:骨への幹細胞移植
著者:藤間保晶, 大串始, 田中康仁, 他.
論文掲載:病理と臨床27(4): 367-371, 2009. -
5. 論文名:Osteogenic activity of bone marrow-derived mesenchymal stem cells (BMSCs) seeded on irradiated allogenic bone培養骨髄由来間葉系細胞による同種壊死骨の骨形成能救済技術の開発
著者:Tohma Y, Dohi Y, Ohgushi H, et al.
論文掲載:Journal of Tissue Engineering and Regenerative Medicine 6(2): 96-102, 2012. -
6. 論文名:再生医療におけるアログラフト
著者:藤間保晶, 大串始, 田中康仁
論文掲載:再生医療における臨床研究と製品開発. 技術情報協会:109-115, 2013. -
7. 論文名:Reg Gene Expression in Periosteum after Fracture and its In Vitro Induction Triggered by IL-6. 骨折後の骨膜に再生遺伝子(Reg遺伝子)が出現することを発見し、インターロイキン6により誘導されることを見出した研究
著者:Tohma Y, Dohi Y, Shobatake R, et al.
論文掲載:Int. J. Mol. Sci. 18(11): 2257, 2017. - ほか多数

渡久地 政尚
Masanao Toguchi
略歴
- 1991年3月
- 琉球大学 医学部 卒業
- 1991年4月
- 医師免許取得
- 1992年
- 沖縄協同病院 研修医
- 1994年
- 沖縄協同病院 外科 勤務
- 2000年
- 癌研究会附属病院 消化器外科 勤務
- 2008年
- 沖縄協同病院 内科 勤務
- 2012年
- 老健施設 かりゆしの里 勤務
- 2013年6月
- 医療法人美喜有会 ふたこクリニック 院長
- 2014年9月
- 医療法人美喜有会 こまがわホームクリニック 院長
- 2017年8月
- 医療法人美喜有会 訪問診療部 医局長
所属学会

圓尾 知之
Tomoyuki Maruo
略歴
- 2002年3月
- 京都府立医科大学 医学部 医学科 卒業
- 2002年4月
- 医師免許取得
- 2002年4月
- 大阪大学医学部附属病院 脳神経外科 勤務
- 2002年6月
- 関西労災病院 脳神経外科 勤務
- 2003年6月
- 大阪大学医学部附属病院 脳神経外科 勤務
- 2003年12月
- 大阪母子医療センター 脳神経外科 勤務
- 2004年6月
- 大阪労災病院 脳神経外科 勤務
- 2005年11月
- 大手前病院 脳神経外科 勤務
- 2007年12月
- 大阪大学医学部附属病院 脳神経外科 勤務
- 2012年3月
- 大阪大学大学院 医学系研究科 修了(医学博士)
- 2012年4月
- 大阪大学医学部 脳神経外科 特任助教
- 2014年4月
- 大手前病院 脳神経外科 部長

加藤 秀一
Shuichi Kato
略歴
- 1997年3月
- 埼玉医科大学 医学部 卒業
- 1997年4月
- 医師免許取得
- 1997年4月
- 三重大学附属病院 整形外科 研修医
- 1998年4月
- 伊賀市立上野総合病院 整形外科 勤務
- 2000年6月
- 鈴鹿中央病院 整形外科 勤務
- 2001年6月
- 三重大学医学部大学院 整形外科学 勤務
- 2003年4月
- 医療法人山本総合病院 整形外科 勤務
- 2004年4月
- 三重県立総合医療センター 整形外科 勤務
- 2006年4月
- 四日市社会保険病院 整形外科 勤務
- 2008年4月
- 医療法人博仁会 村瀬病院 整形外科 勤務
- 2008年9月
- 医療法人美喜有会 理事
- 2009年4月
- 医療法人美喜有会 整形外科みきゆうクリニック 管理者

症例紹介
-
- ひざ関節の症例
- 幹細胞治療の症例
- PRP治療の症例
両変形性膝関節症に幹細胞治療 こちらの患者様は10年前からの左膝関節痛、1年前からの右膝関節痛のため受診していただきました。 南の島で漁師をしている患者様は、仕事で膝へ負担がかかり両膝の痛みが出たと自己分析しておられました。近くの整形外科では両膝の進行期の変形性関節症と診断されました。ヒアルロン酸注射や内服での保存的加療を行ってきましたが、最近は痛みが悪化し漁師の仕事に支障をきたすようになったそうです。 いったん人工関節をしてしまうと耐用性の問題、可動域の問題などから仕事復帰が難しくなるため、選択肢にはないそうです。まだまだ引退することを考えていない患者様は、再生医療を頼って受診されました。 当院の変形性膝関節症の幹細胞治療効果は、末期に至る前の初期、進行期であれば80%から90%の方に満足のいく結果を残せています。末期の方であっても70%から80%の方に満足いただいています。その良好な治療効果は、当院の細胞の質と量へのこだわりによるものと考えています。 当院では冷凍せず幹細胞を培養し、さらに投与する度に培養しているため細胞の生存率は90%以上を誇っています。国内のほとんどのクリニックでは一度に培養して幹細胞を一度冷凍し、投与する際に解凍する方法をとります。それでは解凍する際に幹細胞は大きなダメージを受け、生存率が60%まで低下し、さらに生きている細胞も弱々しいものとなります。 さらに米粒2~3粒程度の脂肪細胞を採取するだけで1億個以上の数まで生き生きとした細胞の培養が可能です。これは、細胞培養時に用いる独自の特殊なシートや繊細な技術をもつ培養師さんのおかげです。一般的なクリニックでは生存率60%の2千万個ほどの幹細胞を投与しているため、実際には生きている細胞は1000万個ほどになってしまいます。一方、当院では冷凍せず培養された生き生きした幹細胞を1億個まで投与可能です。幹細胞の数が多いほど多く軟骨が再生され治療効果が高いということは海外の臨床データで実証済みです。 さらにご自身の血液を用いてかつ不純物や化学薬品を排除して培養しているため、アレルギー反応や副作用の心配もありません。 レントゲン所見 レントゲンでは両膝とも内側関節裂隙の狭小化を認めました。左の方が狭小化は強いです。 <治療効果>左膝に7000万個細胞を計3回、右膝に3000万個細胞を計3回投与+PRP 変形と痛みが強い左膝には7000万個細胞を計3回、右膝には3000万個細胞を計3回投与しました。 その結果、初回投与から3か月後には、左膝は投与前10段階中で9あった痛みが3に、右膝も投与前3であった痛みが0まで軽減しました。 今後も1年ほどかけて、左膝の痛みは軽減していくと思われます。 <治療費> ・関節1部位 幹細胞数 ( 2500万個~1億個) 投与回数 (1回~) 132万円(税込)~ ・PRP治療 16.5万円(税込) <起こりうる副作用> ・細胞採取部の内出血や創部感染、傷跡などが起こることがあります。 ・症状によりMRIやCTなどの検査を受けて頂く事があります。 ID T000139 再生医療医師監修:坂本貞範
2024.04.11 -
- 脳卒中の症例
- 糖尿病の症例
- 肝臓疾患の症例
- 幹細胞治療の症例
糖尿病のHbA1c と肝機能の数値がかなり改善する この方は、糖尿病と肝機能数値が高い状態で、合併症として3年前に脳出血を起こしています。 糖尿病になると、全身の血管に炎症を起こして血管自体が脆くなります。そこに、コレステロールやアルコールなどによってさらに血管が傷つき、結果的に脳血管が敗れてしまい脳出血を起こす原因となるのです。 日本で病院の通院の原因となる疾患の第1位は、高血圧です。高血圧により血管が硬くなって脆くなると、糖尿病や高脂血症になる確率が数倍上がります。このように私たちの血管が脆くなると、色々な病気を発症しやすい環境となるのです。 幹細胞による再生医療では、この方のように異常をきたしている膵臓と肝臓にはもちろん効果はあります。それと同時に、全身の血管に対して傷ついているところも修復及び再生を促します。いくら規則正しい生活を送っていても年齢とともに必ず血管は硬くなります。50代に入ると約6割の方が高血圧症になると言われています。高血圧になると糖尿病、腎臓病、脂質異常症、脳卒中の確率が上がることになります。できるだけ、きれいな状態の血管を維持することが健康の維持につながります。 夢のような話ですが、幹細胞により臓器や血管をきれいにすることが実証されています。当院でも、幹細胞投与により脳血管の詰まりが解放されたり、血管年齢が若返る症例はいくつもみられました。さらに、当院では厚生労働省の許認可により2億個の幹細胞を投与することができます。一般的には1億個の投与となりますが、2億個の幹細胞を投与することで1億個にはみられない高い効果も見られます。幹細胞治療においても、一般の治療と同じで、何事も症状が悪化する前に治療をすることをお勧めします。 投与後の変化 患者様の実際のデータがこちらです。 厚生労働省認可済【2億個の幹細胞】投与を実現 2024年1月より、当院では肝臓疾患に対する点滴において幹細胞数2億個の投与が厚生労働省からの許認可により可能となりました。これにより、従来の幹細胞1億個の投与よりも高い治療効果が期待できるようになりました。 <治療費> 幹細胞点滴 投与回数(1回~) 242万円(税込)~ <起こりうる副作用> ・細胞採取部の内出血や創部感染、傷跡などが起こることがあります。 ・症状によりMRIやCTなどの検査を受けて頂く事があります。 ※こちらでご紹介している症例は一部の患者様です。掲載以外の症例も多数ございます。ご自身の症状については、お気軽にご相談ください。 ID T000722 再生医療医師監修:坂本貞範
2024.04.08 -
- 股関節の症例
- 幹細胞治療の症例
- PRP治療の症例
両変形性股関節症に幹細胞治療 こちらの患者様は9年前からの両股関節痛のため受診していただきました。 9年前に近くの整形外科で変形性股関節症と診断され、3年前には右股関節は軟骨がなくなってしまい、末期の関節症と診断され人工関節を勧められました。「まだ50代と若いので人工関節は早いのでは。今後新たな仕事にも挑戦したい」と、これまで内服薬でしのいでこられました。しかし最近では痛みが悪化し、内服薬の効果も軽減してきました。 さらにレントゲンやMRI撮影をすると右大腿骨頭には骨嚢胞が2つみつかり、「骨嚢胞が潰れると激痛が出現してしまうため、その時は人工関節は避けられない」と主治医に言われたそうです。そこで骨嚢胞を圧壊させない方法や人工関節以外で痛みを取る方法があるのではないかと模索し、当院のホームページに行きつき再生医療へ興味を持たれ受診されました。 骨嚢胞とは、はっきりとした原因はわかっていませんが、変形性関節症において軟骨損傷部位から関節液などが骨に侵入し、骨の溶解が起こり空洞ができた状態のことです。股関節では大腿骨頭と臼蓋の両方に、もしくは一方にできます。小さいものから大きなものまで大きさは様々で、その数も1つだけの場合から沢山できる場合もあります。荷重面に大きな骨嚢胞ができると、体重をかけたときに潰れてしまうことがあります。いったん骨嚢胞が潰れてしまうと、激痛が出現し歩行が困難になります。その場合には人工関節を選択せざるを得なくなります。 患者様は骨嚢胞が圧壊する前に当院を受診していただき私達は安心しました。骨嚢胞が潰れる前であれば、幹細胞を投与すると骨嚢胞が小さくなる症例も数多く経験しておりますので、将来的な人工関節も回避できると考えているからです。これは当院独自の細胞シートによる培養と、冷凍せずにその都度培養する方法によって生み出された強い生命力の幹細胞のおかげであると考えています。この製法の幹細胞であれば個人差はあるもののほとんどの方が長期間、骨嚢胞の圧壊を防ぎ人工関節を回避できてきた治療実績があります。 レントゲン レントゲンでは両股関節の関節裂隙の狭小化を認めましたが、右股関節は左股関節と比べるとかなり狭くなっています。 MRIでは右大腿骨頭の荷重面に2つの骨嚢胞がありますが、幸い圧壊はしていません。 <治療効果>両股関節に1億個細胞ずつ計4回投与+PRP 両股関節に1億個細胞ずつ、計4回投与しました。 初回投与後半年で、右股関節は投与前10段階中6であった痛みが3となりました。左股関節は投与前2であったのが0になりました。 患者様からは「徐々に痛みが楽になっているのがわかります。今後まだまだよくなっていきそうです。来年から新しい仕事に挑戦する自信が湧いてきました。」と話していただけました。 大腿骨頭に骨嚢胞があり、いつ圧壊するのか不安を抱えている方にとっては、幹細胞投与はお勧めの治療法であると考えています。 https://www.youtube.com/watch?v=a6NGuEDLKgQ その他の動画はこちら <治療費> ・関節1部位 幹細胞数( 2500万個~1億個 ) 投与回数( 1回~)132万円( 税込 )~ ・PRP治療 16.5万円( 税込 ) <起こりうる副作用> ・細胞採取部の内出血や創部感染、傷跡などが起こることがあります。 ・症状によりMRIやCTなどの検査を受けて頂く事があります。 ID T000357 再生医療医師監修:坂本貞範
2024.04.05 -
- 肝臓疾患の症例
- 幹細胞治療の症例
点滴投与で痩せやすい体質に!8キロものダイエットに成功 こちらの患者様は15年前に高脂血症を指摘され5年前から内服処方を受けていますが、改善がないと受診されました。さらに内科でエコーやCT検査の結果、脂肪肝とも診断され、食事と運動に気を付けるように指導されました。しかし仕事が忙しく、夜の付き合いも多いため難しいとのことで、再生医療を頼って当院を受診されました。 肝臓は文字通り、内臓の中で一番大きく肝になる臓器です。その働きはたんぱく質、脂質、糖などの栄養素の貯蓄、アルコールや有害物質の解毒・分解、消化酵素である胆汁の生成です。脂肪肝とは、摂りすぎて消費しきれない脂肪や糖質が中性脂肪となり、肝臓の周りにたまった状態のことです。脂肪肝の原因は、食べすぎ、お酒の飲みすぎ、運動不足、肥満が原因と言われています。長時間労働、ストレスフルな人間関係、24時間手軽に食品が購入可能なコンビニの普及、忙しくて運動する時間が取れない現代においては、成人男性の4割が脂肪肝であると言われます。 怖いのは「肝臓は沈黙の臓器」と言われ、脂肪肝、肝硬変になっても症状はありません。脂肪肝だと思って放置していたら知らないうちに肝硬変となり肝臓がんが突然発見される可能性もあるのです。 脂肪肝のように慢性的に肝炎状態が続くと、肝臓の線維化が進み肝臓自体が硬くなり肝硬変となります。線維化した肝臓はもとには戻らないと言われています。脂肪肝・肝硬変に関しては確立された治療法はありません。生活習慣の改善が主な治療法となりますが、これ以上肝臓が線維化しないようにといった予防的な意味合いしかありません。現在の保険診療の治療では、脂肪肝も肝硬変も根本的な治療はありません。当院ではそういった肝障害に対して再生医療を提供してきました。 具体的には、下腹部から採取した脂肪細胞から幹細胞を分離・培養し、幹細胞のホーミング効果を期待して静脈から点滴します。ホーミング効果とは、体内に入った幹細胞が再生を必要としている部位・組織から放出されるシグナルを見つけ出し、その部位・組織に自動的に集まり、目的の細胞に分化したり、傷んだ部位・組織を修復することです。 肝臓の再生医療においては、投与された幹細胞は肝臓の炎症や線維化して硬くなってしまった組織を発見し、溶解・修復させることです。よって点滴する幹細胞は生きていないとホーミング効果が期待できません。当院で使用する細胞は、冷凍保存せず投与するたびに培養しているため2回目、3回目に投与する細胞も生存率90%以上の生き生きとしたフレッシュな細胞です。幹細胞に十分なホーミング効果を発揮してもらうには点滴する幹細胞の数も重要と考えています。 当院の細胞培養は細胞培養技術がトップクラスの施設と提携して行うため、米粒2~3粒程度の脂肪細胞を採取するだけで1億個以上の数まで細胞培養が可能です。2024年からは、厚生労働省より2億個の幹細胞を投与できる許認可を得ることができました。これにより従来の1億個の幹細胞より高い効果が期待できます。 CT初見 腹部CTでは肝臓は脾臓よりも黒っぽく脂肪肝であることがわかります。 投与後の変化 1億個細胞を計5回点滴投与しました。 5回目の血液検査で、投与前はγ―GTP128であったのが投与後には68まで低下、中性脂肪は634であったのが340まで低下しました。 患者様からは「薬を飲んでも中性脂肪は減らなかったのが半減してうれしい。痩せやすい体質になって8キロものダイエットに成功したのもうれしい。」と喜んでいただけました。 痩せやすい体質と前向きな気持ちを手に入れダイエットに成功し、患者様にはこれからも健康増進に取り組んでいただけそうで私達もうれしく思いました。 厚生労働省認可済【2億個の幹細胞】投与を実現 2024年1月より、当院では肝臓疾患に対する点滴において幹細胞数2億個の投与が厚生労働省からの許認可により可能となりました。これにより、従来の幹細胞1億個の投与よりも高い治療効果が期待できるようになりました。 <治療費> 幹細胞点滴 投与回数(1回~) 242万円(税込)~ <起こりうる副作用> ・細胞採取部の内出血や創部感染、傷跡などが起こることがあります。 ・症状によりMRIやCTなどの検査を受けて頂く事があります。 ※こちらでご紹介している症例は一部の患者様です。掲載以外の症例も多数ございます。ご自身の症状については、お気軽にご相談ください。 ID T000393 再生医療医師監修:坂本貞範
2024.03.25
坂本理事長のブログ
藤間院長のブログ
スタッフブログ
トピックス
-
- 内科疾患、その他
- 内科疾患
高血圧の原因に女性ホルモンの影響が?更年期高血圧と対策 高血圧は生活習慣病の一つとして広く知られています。 しかし、高血圧の裏に別の病気が隠れていることも少なくありません。 思い当たる節がないのになぜか血圧が高いと思ったことは?その高血圧は、更年期によるものかもしれません。 40〜50代の女性が注意すべき更年期高血圧! 更年期は、女性ならば誰しもが通る道です。そして、更年期の変化によって生じる高血圧を『更年期高血圧』と呼びます。 これまで健康診断に引っかかることもなかったのに急に血圧が上がったり、生活習慣には気をつけているのに血圧を測ったら高かった、などということがあれば要注意!高血圧を放置しておくと、心臓病や脳血管障害に繋がることも。だからこそ、病態を理解し、きちんと対策を行うことが大切なのです。 女性ホルモンの減少によって生じる更年期障害 更年期とは、閉経前後それぞれ5年間を合わせた10年間を指します。日本人の平均閉経年齢は50歳とされていますが、早い人では40代前半、遅い人では60歳手前で迎えることもあります。 女性ホルモンには、エストロゲンとプロゲステロンの2種類があります。更年期になると卵巣機能が低下し、それに伴い女性ホルモンも減少します。このうちエストロゲンの減少が、更年期障害の発症に大きく関わっていると考えられています。ホルモンバランスを保てなくなったことに対して、脳がそれを補おうと必死に身体に様々な命令を送り、それが更年期症状として現れてしまうのです。 加えて、加齢による身体的影響、性格などの心理的影響、そして家庭や仕事によるストレスが複雑に絡み合い、個々の症状に現れてきます。症状が軽度で済む人もいれば、日常生活に支障が出るくらい重い症状を呈する人もいます。軽度の場合は更年期症状、症状が重くなると更年期障害となります。 多岐にわたる更年期症状ですが、高血圧以外には以下のようなものが挙げられます。 ・血管運動に関わる症状(ほてり、のぼせ、発汗、動悸、息切れ、むくみなど) ・精神症状(頭痛、不眠、不安感、イライラ、抑うつ、無気力など) ・消化器症状(便秘、下痢、胃の不快感、胸焼け、吐き気など) ・皮膚症状(かゆみ、湿疹など) ・運動器に関わる症状(肩こり、腰痛、背中の痛み、関節痛など) ・そのほか(疲労感、排尿トラブル、不正出血など) それでは、どうして更年期になると血圧まで影響を受けてしまうのでしょうか? 更年期障害と高血圧の関係性 日常生活の中で、例えば運動中は血圧は上がり、運動後リラックスしている時は血圧が下がります。このように血圧の変動がある中でも、私たちの身体は常に一定の血圧を保持できるよう機能しており、高くなりすぎず、低くなりすぎないように調整されているのです。エストロゲンは、その調整因子の一つであり、血管を広げて血圧を下げる働きをします。よって、このエストロゲンが減少すると、血圧は下がりにくくなってしまうのです。 さらにもう一つ、自律神経の乱れも更年期高血圧に大きく関わる因子です。 自律神経には、交感神経と副交感神経があります。活発な時や緊張状態の時には交感神経が優位となり、血管が収縮して血圧が上昇します。逆に休んでいる時や就寝中は副交感神経が優位となり、血管は拡張して血圧が下がります。このように血圧調整にとても大切な自律神経ですが、女性ホルモン低下に追いつけないことでパニックになった脳は、様々な命令を身体に送る中で、自律神経も乱してしまうのです。自律神経の乱れは血圧変動のみならず、上記に述べたような精神症状や身体症状の原因にもなっています。 このようにエストロゲンの減少と自律神経の乱れは、本来私たちに備わっている血圧調整の歯車を狂わせ、結果的に更年期高血圧を引き起こすのです。 更年期高血圧の特徴とは? 更年期高血圧の特徴は、大きく2つあります。 特徴①40〜50代の女性に生じる まず一つ目の特徴として、更年期に生じる高血圧のため、40〜50代の女性にみられます。 それゆえ、更年期を過ぎると血圧も正常化する人もいます。しかし、加齢とともに血管の老化や自律神経の働きが低下し、更年期高血圧以降もそのまま高血圧に転じてしまう人も多くみられます。 特徴②血圧が不安定になり上がりやすくなる 二つ目の特徴は、血圧が不安定になることです。 不安やイライラなどの精神的な要素も加味され、ちょっとしたことで血圧が上がりやすくなっています。 更年期高血圧に!今日から自分でできる対策 今日からご自身で始められる、更年期高血圧の対策をいくつかご紹介いたします。 血圧の管理 まずは血圧の測定を定期的に行い、その血圧上昇が一時的なものなのか、あるいは血圧が高い状態がずっと続いているのか、そしてどのような時に高くなるのか確かめることが大切です。 ①血圧上昇が一時的なものなのか ②血圧が高い状態がずっと続いているのか ③どのような時に高くなるのか 外出時は精神的・身体的・環境的因子が血圧に影響することも多いため、自宅での血圧測定を推奨します。 定期測定は起床時と就寝前の2回、そしてめまいや動悸など、上記の更年期症状が出た際にも血圧を測定し、血圧の推移とともにどのような状況で血圧が上がったのかを書き留めておくと良いでしょう。自分の状況を把握するだけでなく、医師からの診察の際にも役立ちます。 生活習慣の見直し 次に、生活習慣の見直しも行いましょう。 冒頭で述べたとおり、塩分過多は高血圧の原因として最も多いとされています。また、肥満は動脈硬化を起こし、結果的に高血圧を生じさせます。 近年は特に、インスタント・冷凍食品の改良化や食事のデリバリー事業などが進み、便利な時代となりました。しかし、既製品や外食は塩分量とカロリーが高くなる傾向にあります。減塩調味料の使用や野菜多めの食事を心がけるようにしましょう。 運動習慣を取り入れる 仕事や家事に追われて、なかなか運動の時間をとるのが難しい方もいらっしゃいます。 1日の中で、少し早起きしてウォーキングをしてみる、パソコンで10分のエクササイズ動画を真似してみる、休日にスポーツセンターに行ってみる、一駅分歩いてみる、など小さなことで構いません。 ・ウォーキング ・10分簡易エクササイズ ・休日にスポーツセンターに行く ・一駅分歩いてみる 自分のできる範囲内で何か始めてみましょう。運動するとイライラした気持ちも収まり、身も心もスッキリします。 睡眠時間を十分に取る 睡眠不足は交感神経を刺激し、高血圧を引き起こします。 しかし更年期障害の一つに不眠もあり、夜なかなか寝付けない人もいるかと思います。 就寝時間の2時間前までに食事を摂り終える、布団に入ったらスマートフォンはいじらない、夜にカフェインやお酒は控える、リラックス効果のある香りや音楽をつけてみる、などの工夫をおすすめします。 ・就寝時間の2時間前までに食事を摂り終える ・布団に入ったらスマートフォンはいじらない ・夜にカフェインやお酒は控える ・リラックス効果のある香りや音楽をつける ここまでの対策は自身でできることですが、やはり重症化予防の鍵は早期受診となります。 高血圧を適切に治療しなかった場合、心臓や脳の血管に障害を生じ、心筋梗塞や脳卒中のリスクが上がります。こうならないためにも、適切な治療を受けることが必要です。 循環器内科を受診しましょう 更年期症状の改善に関しては婦人科、高血圧の改善に関しては循環器内科が専門となります。今はオンライン診療やオンライン医療相談も広まってきていますので、コロナ以降病院から足が遠のいた方や忙しい人も医師に気軽に相談できます。気になる方はぜひ調べてみてください。 治療法としては、上記に述べたような生活指導の上、高血圧に対しては降圧薬、更年期症状に対しては漢方薬や抗精神薬の処方、そして必要な場合にはホルモン療法なども検討されます。 どの治療法になるかは、患者さん一人一人に合った方法を医師と相談して決めていくこととなります。 まとめ・高血圧の原因に女性ホルモンの影響が?更年期高血圧と対策 更年期高血圧は、更年期の身体の変化に伴って生じるものです。 更年期を過ぎると血圧が元に戻ることもありますが、多くの場合は加齢の変化と相まって高血圧のままとなります。命を脅かす病気に発展しないよう、血圧のコントロールを行うことが大切です。 毎日の生活習慣を改善し、早期に病院を受診しましょう。このコラムがご参考なれば幸いです。 No.3 監修:医師 渡久地 政尚 参考文献 日本高血圧学会. 高血圧治療ガイドライン2019.
最終更新日:2024.04.22 -
- 内科疾患、その他
- 内科疾患
高血圧の原因と対策:食事・運動・薬で血圧をコントロール! 高血圧を放置すると、脳卒中・心疾患・腎臓病など命に関わる病気に発展するリスクがあります。 それにもかかわらず、国内で4300万人と推計されている高血圧罹患者のうち、適切に血圧がコントロールされているのはたった3分の1といわれています。残りの3分の2の方はコントロール不良あるいは未治療、その半数は自分が高血圧であることに気づいてすらいないのです。 高血圧は、気がつかない間に重大な病気を引き起こし得るため「サイレントキラー」とも呼ばれています。 血圧を予防・改善するには何をすれば良いのでしょうか。鍵となるのは、食事や運動の見直しと適切な薬の使用です。 本記事では、高血圧の原因や症状、基準値などの基本的な情報とともに、効果的な対策法を分かりやすく解説していきます。 高血圧の原因:何が血圧を上げるのか 高血圧には、原因別に「二次性高血圧」と「本態性高血圧」の2つがあります。 二次性高血圧 二次性高血圧は、特定の病気が原因で血圧が上昇するケースを指します。 その病気を治療することで、高血圧を改善することが期待できます。 本態性高血圧 本態性高血圧は、複合的な原因で起こる高血圧のことです。 原因として、体質や遺伝的な要素に加え、不適切な食事・肥満・運動不足・喫煙などの生活習慣が関係しています。特に日本人は塩分をとりすぎやすく、血圧上昇に大きな影響があります。血圧を下げるためには減塩をはじめとした生活習慣の改善に取り組むことが必要です。 二つの高血圧のうち、圧倒的に多いのは本態性高血圧です。この記事でも主に本態性高血圧について取り上げていきます。 高血圧診断の基本:血圧の基準値とは 高血圧の診断は、診察室と自宅それぞれの血圧測定で行います。 まず、診察室血圧が【収縮期血圧140/拡張期血圧90mmHg以上】であれば自宅で血圧を測定します。 自宅の血圧が135/85mmHgであれば高血圧の確定診断となります。自宅での血圧が測定できない場合は、診察室の血圧だけでも「高血圧」と考えます。 では、基準値より低ければ正常かというと、そうではありません。 「正常血圧」は、診察室で測った血圧が120/80mmHg未満、自宅で測った血圧が115/75mmHg未満の場合を指します。 高血圧でなくても、正常血圧より高いグレーゾーンがあるのです。 病院での測定値で120〜139/80〜89mmHg、家庭血圧で115〜134/75〜84mmHgの場合が、以下のグレーゾーンに該当します。 日本高血圧学会 高血圧治療ガイドライン2019 高血圧の診断を満たさなくとも、血圧が高くなるにつれて脳卒中や心筋梗塞などの「脳心血管の疾患」が起こりやすいのです。また、グレーゾーンの方は生涯のうちに「高血圧」の基準を満たす可能性も高くなります。たとえ診断に至らなくても、血圧が高めの方は対策をとることをおすすめします。 また、自宅での血圧が高血圧に該当しなくても、診察室血圧のみが高血圧の基準を満たす場合は「白衣高血圧」といいます。白衣高血圧の方は高血圧でない方と比べて、将来的に脳心血管の病気を起こすリスクが高く、要注意です。 自宅血圧測定の方法:計測する時間帯や回数 自宅で血圧を測定することは高血圧の診断に重要ですが、それ以上に治療においても重要です。家庭血圧が降圧治療の一番の指標になるからです。 家庭血圧が正常範囲になることが、高血圧対策が有効と判断する重要な材料になるのです。高血圧と診断されたら、家庭血圧を毎日測って把握することが治療の第一歩になります。 では、家庭で血圧を測定するときのポイントをお伝えします。 家庭血圧測定のポイント 1日の中で血圧を測る時間帯は、朝と夜の両方が基本です。 朝は起きてから1時間以内に測ります。朝ごはんを食べる前、薬を飲む前、トイレに行った後が良いです。測定前に水分を取ることは構いません。夜は寝る前に測定しましょう。 血圧測定時は、1〜2分間じっと落ち着いてから行います。測る前に深呼吸をしてリラックスしましょう。急いで測定すると、正確な血圧が出ないことがあります。 血圧は原則的には、都度2回測定します。2回の結果はどちらとも記録しておきましょう。つまり、朝2回、夜2回、計4回測ります。 ・起床後 1時間以内に2回 ・就寝前 2回 2回測った血圧の値は、平均値で判断します。 高血圧の診断や治療の効果を判断するには、5〜7日間の血圧の平均値を用います。そのため、毎回の血圧測定毎に「高かった」「今日は下がっている」と一喜一憂することはあまり意味がありません。 家庭血圧測定で一番大事なことは、継続することです。ご自身の生活スタイルに合わせて測定時間を調整しましょう。 毎日決まった時間に測定することが望ましいですが、難しい場合は担当医師と相談しましょう。 高血圧の自覚症状:頭痛・肩こりとの関係と潜むリスク 高血圧のみでは自覚症状がないことが多いです。人によっては、血圧が高いと軽度の頭痛や肩こりなどの症状が現れることがあります。 一方で、血圧が急激に高くなることによって危険な状態に陥る場合もあります。それが、「高血圧緊急症」です。脳・心臓・腎臓・大きな血管などに障害をきたし、放置すると命に関わります。多くの場合は180/120mmHg以上の高度の血圧上昇により起こるものです。 高血圧緊急症の例として次のような疾患が含まれます。 〈高血圧緊急症の例〉 ・高血圧性脳症 ・脳卒中 ・急性大動脈解離 ・急性心不全 ・急性心筋梗塞 ・急性腎不全 とくに「高血圧性脳症」は耳慣れない言葉かもしれません。これは急激に血圧が上昇することで、脳の血流が必要以上に増加して脳が浮腫む病気です。 そのまま放置すると脳出血や昏睡状態を引き起こし、命を落とす危険性もあります。急激な血圧上昇に加えて悪化する頭痛・吐き気・嘔吐・視力の障害・意識障害・痙攣などの症状は、緊急で受診をする必要があります。 高血圧対策!生活習慣の見直しで予防・改善 高血圧は生活習慣病であり、生活習慣を見直すことは高血圧の予防・改善の第一歩です。 具体的に改善すべき点の例を以下に挙げます。 〈高血圧対策において見直すべき項目〉 ・塩分を控えめにしているか ・食事の内容は野菜・果物・魚などが中心になっているか ・運動習慣があるか ・適正な体重(BMI 25kg/m2未満)を維持できているか ・お酒を飲み過ぎていないか(エタノールで男性20-30mL/日、女性で10-20mL/日以下) ・タバコを吸っていないか このような項目の1つだけを見直したとしても、一気に10mmHgも20mmHgも血圧が下がるものではありません。2つ、3つと可能な限り複数合わせて取り組むことで、大きな効果をもたらす可能性はあります。 降圧薬を飲んでいる状況でも、生活習慣を改善することで薬を減らすことができるかもしれません。それぞれの項目は互いに関連することも多いため、まずはできることから始めましょう。 食事療法で高い血圧を下げるには 食事で血圧を下げるのに重要な見直しポイントは「減塩」と「食事の内容と量」です。また、「飲酒量のコントロール」も大切です。一つずつ解説していきましょう。 減塩について 減塩については、1日に6g未満を目標にします。 厚生労働省の令和元年国民健康・栄養調査によると日本人の1日の塩分摂取量の推定は10.1gとされています。多くの日本人にとって6g未満の塩分量の食事は、非常に薄味に感じるでしょう。 医療機関では尿検査で1日の塩分量の推定をすることができます。あくまで推定値で誤差はありますが、自分がどのくらいの塩分を取っているかを知る一つの目安になるでしょう。気になる方はかかりつけの病院や最寄りの医療機関で相談してみてください。 減塩のポイントは、出汁やスパイス、お酢などを上手に使うことです。また、漬物やハム・ソーセージなどの加工食品には食塩が多く含まれるためなるべく避けましょう。味噌汁やラーメン・うどんなどの汁は飲み干さないようにしましょう。 食事の内容と量 食事の内容としては、野菜・果物・魚を積極的に摂り、肉類など動物性の脂肪は控えめにすることが重要です。 野菜や果物に多く含まれるカリウムは、塩分に含まれるナトリウムを排出させて血圧を下げることがわかっています。ただし、カリウム摂取については腎臓病の方は制限が必要な場合もあります。通院中の方は主治医の指示に従いましょう。 肉や高脂肪の乳製品などに多く含まれる飽和脂肪酸が過多になると、血中のコレステロールが増え動脈硬化が進み血圧が上がります。一方で魚や多くの植物性油脂・ナッツなどに多く含まれる不飽和脂肪酸は悪玉コレステロールを減らし血圧を下げる効果があります。 そして、どんなに体に良い食事でも食べ過ぎは肥満のもとです。肥満は血圧上昇とも関連するため、腹八分目を心がけましょう。 飲酒量のコントロール 飲酒習慣は血圧を高くします。節酒も血圧を下げるのに有効です。高血圧がある方の飲酒量の目安は1日あたりエタノールで男性20-30mL、女性で10-20mL以下です。 〈1日あたりの飲酒量の目安:男性の場合〉 ・日本酒 1合 ・ビール 中瓶1本 ・焼酎 0.5合 ・ウイスキー ダブル(約60mL) ・ワイン 2杯 ※女性はこの半分を目安にしてください。 運動療法で血圧は下がる?有酸素運動のすすめ 運動は血圧を下げるだけでなく、肥満、糖尿病、脂質異常症などの生活習慣病の予防や改善にも効果があります。 おすすめは有酸素運動です。ウォーキングやジョギング、エアロビクス、水泳など、ご自身が楽しいと感じるものが継続しやすいでしょう。 「少しきつい」と感じるくらいの運動強度で、1日30分以上行ってください。 毎日続けるのが難しい場合は、まずは週に1回でも運動習慣を作るところから始めてみてはいがかでしょうか。 薬物療法による高血圧のコントロールについて知ろう 高血圧の薬は様々な作用機序で血圧を下げます。代表的なものは以下の通りです。 〈代表的な降圧薬〉 ・ACE阻害薬、ARB:血圧を上昇させるホルモンの作用を阻害する ・カルシウム拮抗薬:血管を拡張させて血圧を下げる ・利尿薬:余分な水分やナトリウムを体外に排出する ・β遮断薬:交感神経を抑制して心収縮を抑えて血圧を下げる 血圧を下げる薬を内服する上で大切なことがあります。それは、血圧が下がっても自己判断で中止しないことです。 どのような降圧薬であっても、効果はあくまで一時的なものです。飲み始めて血圧が下がったからと内服をやめてしまうと、元の高い血圧に戻ってしまいます。ただし、生活習慣を良くしていくことで徐々に減薬・中止をすることができる方もいます。薬の調整は、運動や食事に気をつけながら、家庭血圧の測定結果をもとに医師と相談して決めましょう。 まとめ・高血圧は食事・運動・薬でコントロール! 本記事では高血圧の原因をはじめとした基本情報と、効果的な対策について説明しました。 高血圧の多くを占める本態性高血圧では、塩分のとりすぎをはじめとした生活習慣が大きく関わっています。症状がないからといって高血圧を放置すると、命に関わる病気を起こしかねません。 血圧が気になるのであれば、まずは定期的な血圧測定を行い日々の血圧がどのくらいかを把握するように努めましょう。 高血圧対策の第一歩は生活習慣を改善することです。一気に運動も食事も改善しようとするのが難しければ、できるところから始めてみましょう。薬をすでに処方されている方は、しっかり続けることもお忘れなく。 本コラムが皆様の健康的な生活に少しでも役立ちますと幸いです。 No.2 監修:医師 渡久地 政尚 参考文献 厚生労働省 令和元年国民健康・栄養調査報告 日本高血圧学会 高血圧治療ガイドライン2019
最終更新日:2024.04.24 -
- 内科疾患、その他
- 内科疾患
高血圧とストレスの深い関係とは?予防法や改善策を解説! 高血圧は世界中で数多くの人々が罹患している疾患です 。この病気は、しばしば「サイレントキラー」と呼ばれています。 高血圧自体では症状がほとんどないにもかかわらず、心臓病や脳卒中など、命に関わる病気のリスクを高めるからです。高血圧とストレスの関係は、多くの研究で注目されており、これら二つの間には密接なつながりがあることがわかっています。 本記事では、ストレスと高血圧の関係を深く掘り下げ、予防や改善のために役立つポイントを解説していきます。 高血圧とは何か 高血圧は、血液が血管壁にかかる圧力が通常よりも高い状態を指します。この状態が継続すると、心臓や血管に過剰な負担がかかり、心臓病、脳卒中、腎臓病などの重大な健康問題のリスクが高まります。 血圧は「収縮期血圧」と「拡張期血圧」の二つの数値で表されます。 収縮期血圧(上の数値)は、心臓が収縮して血液を体中に送り出す際の圧力です。拡張期血圧(下の数値)は、心臓が次の収縮に備えて血液で満たされる際の圧力です。 通常、成人の正常な血圧は収縮期が120mmHg未満、拡張期が80mmHg未満とされています。一方、高血圧は、収縮期血圧が130mmHg以上または拡張期血圧が80mmHg以上である状態を指します。 引用) 高血圧治療ガイドライン 2019(JSH2019)作成委員会 p18 血圧は、測定する場所によって以下のように分けられています。 診察室血圧 医療機関で医療従事者が測定する血圧です。 この環境で高い血圧を示す人は、白衣高血圧の可能性があります。 白衣高血圧は、医療機関での血圧測定時にのみ高血圧の値が出る状態を指します。家庭での測定や、24時間血圧モニタリングでは正常範囲内の血圧値を示すにも関わらず、医療機関で測定すると血圧が上昇する現象です。この現象は、医療環境に対する不安や緊張が原因で起こります。 白衣高血圧自体が直接的な健康リスクを示すわけではありませんが、一部の研究では、白衣高血圧の人が将来的に持続的な高血圧(本態性高血圧)を発症するリスクが高まる可能性が指摘されています。したがって、白衣高血圧の診断を受けた場合でも、定期的な血圧のモニタリングと、必要に応じたライフスタイルの見直しが推奨されます。 家庭血圧 患者自身が自宅で測定する血圧です。 日常生活における血圧の変動をより正確に把握できる方法とされています。 24時間血圧モニタリング(ABPM: Ambulatory Blood Pressure Monitoring) 24時間持続的に血圧を測定する方法で、患者が通常の日常生活を送りながら測定します。 昼夜の血圧の変動や、睡眠中の血圧を含めた全体的な血圧コントロール状態を評価するのに有用です。 高血圧2つのタイプと原因 高血圧には二つの主なタイプがあります。「一次性高血圧」(原因不明の高血圧)と「二次性高血圧」(特定の原因による高血圧)です。 一次性高血圧 ほとんどの高血圧はこのタイプに該当し、明確な原因は特定されていませんが、遺伝、食生活、生活習慣など複数の要因が関連していると考えられています。 二次性高血圧 何らかの病気や状況が原因で血圧が高くなるケースです。腎臓病、内分泌異常、特定の薬剤の使用などが原因で起こります。 高血圧のリスク要因として考えられること 高血圧のリスクを高める要因には、以下のようなものがあります。 ①年齢 加齢とともに血圧は高くなる傾向にあります。 ③性別 年齢によっては、 男性の方が女性より 高血圧になりやすい時期があります。 ③家族歴 高血圧の方が家族に多い場合、遺伝的要因が関係している可能性があります。 ④不健康な生活習慣 不健康な食事(特に塩分の過剰摂取)、運動不足、肥満、過度のアルコール摂取、喫煙などが高血圧のリスクを高めます。 ⑤ストレス ストレスと緊張も高血圧のリスクを高める重要な因子です。 なぜストレスがかかると血圧が上昇するのか? さて、血圧が上がる原因の一つに、ストレスがあると述べました。 ストレスは、私たちの身体に多方面から影響を及ぼします。その影響の一つとして、身体の自律神経系を活性化させることで心拍数と血圧を一時的に上昇させることが知られています。これは、身体が直面した脅威に対処するための「戦うか逃げるか」の反応として機能し、短期間であれば自然な生理現象とみなされます。しかし、この反応は短期的なものに留まらず、ストレスが慢性化すると、高血圧を引き起こす原因となり得ます。 慢性的なストレスは、心拍数と血圧の持続的な上昇を引き起こし、高血圧につながる恐れがあります。加えて、ストレスが多い環境にいると、健康に良くない食生活や運動不足など、高血圧につながる他のリスク要因が増えがちです 。不健康な生活習慣は、さらに血圧に悪影響を及ぼし、悪循環を生むことになります。 ストレスは交感神経を刺激し、一時的な心拍数と血圧の上昇を引き起こすだけでなく、長期間にわたるストレスの影響で高血圧症を引き起こす可能性があることが分かります。 そして、慢性的なストレスが不健康な生活習慣へと導くこともあり、さらに血圧に悪影響を及ぼす可能性があるのです。したがって、ストレス管理は血圧コントロールの観点からも非常に重要であると言えます。 高血圧を引き起こす主なストレス源 職場や家庭内でのストレスは、高血圧の主な原因の一つとして広く認識されています。 職場での過剰な負担や、期限の迫ったプロジェクト、職場内での対人関係の問題などが、ストレスレベルを高める主な要因となるのです。これらのストレスは、交感神経系を刺激し、心拍数の増加や血管の狭窄を引き起こし、結果的に血圧を上昇させる可能性があります。 家庭環境も、ストレスの大きな源となり得ます。家族間の不和、経済的な問題、子育てや介護などの責任は、個人のストレスレベルを大幅に高めることがあります。家庭内でのストレスは、しばしば外部には見えにくいため、解決されずに長期化することがあります。このようなストレス状態が続くと、心身の健康に悪影響を及ぼします。特に血圧に関しては、その数値を大幅に高めるリスクになります。 さらに、職場と家庭の双方からのストレスは、不健康な生活習慣につながってしまうことがあります。過剰なストレスは、不安や抑うつといった精神的な問題を引き起こすことがあり、これが不健康な食事、運動不足、過度のアルコール摂取や喫煙など、高血圧に直結する生活習慣へつながるのです。したがって、職場や家庭内でのストレス管理は、血圧を健康的なレベルに保つために、 極めて重要です。 血圧測定時にストレスの影響で血圧は変動するのか 病院や自宅で測定した血圧が正常でも、職場や家庭のストレスにさらされている昼間の時間帯の血圧が高くなることがあります。具体的には、135/85mm/Hg以上の場合、昼間高血圧と定義されています。 精神的・身体的なストレスは血圧に影響を与えることが知られています。 健康診断の際や病院での血圧は正常でも、ストレス状況にある職場で測定した血圧が高い「職場高血圧」は、肥満の方や高血圧の方が家族にいる方に多いという特徴があります。 高血圧の治療 -生活指導や薬物療法 - 高血圧治療は、患者のライフスタイルの見直しと薬物療法を組み合わせていきます。 ①ライフスタイルの改善 高血圧治療においては、生活習慣の改善が重要となります。例えば、食生活、運動習慣、禁煙、ストレス管理など、日々の生活習慣を見直していきます。 具体的には、塩分の過剰摂取を避け、果物や野菜を豊富に含むバランスの取れた食事を心がけ、規則正しい運動を行うことが推奨されます。 具体的な食塩摂取量は、「健康日本21(第三次) 」の目標値では7g未満、「日本人の食事摂取基準(2020年版)」の目標量では、成人男性で7.5g未満、成人女性で6.5g未満とされています。 成人男性・・・7.5g未満 成人女性・・・6.5g未満 また、日本高血圧学会では、高血圧患者における減塩目標を1日6g未満にすることが勧められています。 禁煙や適度なアルコール摂取、良好な睡眠習慣、効果的なストレスマネジメントが血圧管理に役立ちます。 ②薬物療法 生活習慣の改善だけでは血圧がコントロールできない場合、薬物療法が検討されます。 血圧降下薬には、ACE阻害薬、アンギオテンシンII受容体ブロッカー(ARB)、カルシウムチャネルブロッカー、利尿薬、βブロッカーなどがあります。 患者の状態や既往症などを医師が総合的に判断することによって、こうした薬剤は個別に処方されます。 ストレスによる高血圧の予防法 - 生活習慣の改善 - 上記で述べたように、ストレスは高血圧と深い関係があります。そこで、ストレスに上手く対処することが血圧の上昇を防ぐために重要となります。 ここでは、ストレスへの対処法と改善策を詳しくご紹介します。 規則正しい運動 軽い有酸素運動は、ストレスを軽減し、血圧を低下させるのに役立ちます。週に数回、歩行やジョギング、水泳などを行うことをお勧めします。 バランスの取れた食事 塩分の摂取量を控えめにし、果物、野菜、全粒穀物を豊富に含む食事を心掛けてください。これらは血圧を健康的なレベルに保つのに役立ちます。 十分な睡眠 良質な睡眠はストレスレベルを低下させることができます。毎晩7〜8時間の睡眠を目指しましょう。 禁煙と節度ある飲酒 喫煙と過度の飲酒は血圧に悪影響を及ぼします。これらの習慣を控えることで、血圧の管理に役立ちます。 深呼吸や瞑想 深呼吸や瞑想は、ストレスを感じたときに交感神経の活動を鎮め、リラックス効果を促進します。日常生活にこれらの練習を取り入れることで、ストレスレベルを下げることができます。 趣味や興味の追求 好きな活動や趣味に時間を割くことで、心のリフレッシュが可能となり、ストレス軽減につながります。 社会的サポート 友人や家族との良好な関係は、ストレスの軽減に非常に重要です。信頼できる人と感情を共有することで、ストレスを効果的に管理できます。 ストレスに上手く対処することが、血圧の上昇を防ぐために重要です。日常生活にぜひ取り入れてみましょう。 まとめ・高血圧とストレスの深い関係とは?予防法や改善策を解説! 高血圧とストレスは、現代社会において無視できない健康問題です。しかし、適切なストレス管理、健康的な生活習慣、そして必要に応じて医療機関を受診し、治療を受けることによって、これらのリスクを大幅に軽減することが可能です。 生活の中で意識的にリラックスする時間を作り、バランスの取れた食事と定期的な運動を心がけましょう。 この記事がご参考になれば幸いです。 No.1 監修:医師 渡久地 政尚 参考文献 高血圧 | e-ヘルスネット(厚生労働省) 高血圧治療ガイドライン 2019(JSH2019)作成委員会 p18 高血圧治療ガイドライン 2019(JSH2019)作成委員会 p15 高血圧:診断と治療の進歩 トピックスI.診断と病態 1.本態性高血圧の成因.日本内科学会雑誌.2007;96(1):4-8. 高血圧治療ガイドライン 2019(JSH2019)作成委員会 p21 高血圧治療ガイドライン 2019(JSH2019)作成委員会 p22 健康日本21(第三次) 厚生労働省 国民の健康の増進の総合的な推進を図るための基本的な方針 p28
最終更新日:2024.04.22 -
- 幹細胞治療
- 脊椎
- 脊椎、その他疾患
リゾトミーの術後後遺症にお悩みですか?日帰り最新医療のご紹介 「つらい腰痛が良くなると思って受けた治療で、後遺症が残ってしまった…」 そのようなお悩みはありませんか。 リゾトミーは慢性難治性腰痛の原因となる神経の伝達を遮断し、痛みをとる方法です。体への負担は少ないとされていますが、医療行為である以上は合併症のリスクからは逃れることはできません。人によっては後遺症が長期化してしまうこともあり得ます。 この記事では腰痛に対するリゾトミーの詳細と、術後後遺症としての神経損傷について、そして最新の後遺症治療の「再生医療」について解説しています。 リゾトミーとは?高周波熱凝固療法について解説 リゾトミーは英語でrhizotomyと表記されます。主に脊椎の椎間関節の痛みをもたらす脊髄神経後枝内側枝に対する「高周波熱凝固療法」のことです。整形外科、あるいは疼痛を専門に扱う「ペインクリニック」で行われています。 この高周波熱凝固療法の技術は、従来の神経ブロック注射で効果が一時的である慢性難治性疼痛に用いられるものです。リゾトミーにおいては、椎間関節ブロックによって痛みが椎間関節由来だと診断された場合に行われます。 高周波熱凝固療法では、ターゲットの神経のすぐ近くにピンポイントで高周波(ラジオ波)を流す特殊な針を刺します。ラジオ波が神経組織内のイオン分子を高速振動させることで神経組織が高温となり、熱で変性するのです。変性した神経は痛みを伝える機能を失うため、痛みを感じなくなります。 このような治療は、神経の伝達を遮断して痛みが脳に伝わらないようにするため「神経破壊法」と呼ばれています。神経を破壊するというと恐ろしく感じるかもしれませんが、長期に痛みを抑える優れた治療法として広く用いられています。 従来、リゾトミーはX線による透視画像を見ながらターゲットになる神経を探していました。一部の施設では内視鏡の技術を用いたリゾトミーも行われています。この内視鏡とは、坐骨神経痛などを起こす椎間板ヘルニアの手術(PELD手術)で実用化されているものです。内視鏡で直接ターゲットの神経を探すことで、より確実にねらった神経を遮断することができます。日本国内ではまだできる病院やクリニックは少ないですが、今後普及していくかもしれません。 リゾトミーの術後合併症としての神経損傷 リゾトミーの合併症のうち、後々まで問題になりやすいのが神経損傷です。滅多に起こることではありませんが、神経を傷つけてしまうことで、痺れ・痛み・麻痺などが起こります。 傷ついた神経の再生は非常に困難です。確実に治るという治療法はなく、内科的な投薬治療やリハビリテーションを組み合わせて自然に良くなるのを待つことになります。徐々に改善する方もいますが、症状が長期にわたって続く場合も残念ながら存在します。 術後後遺症に再生医療 術後後遺症が良くならない場合の選択肢として、「幹細胞治療」が期待されています。幹細胞治療とは、臓器や器官の機能再生を目的とした再生医療のひとつです。 幹細胞治療では、「幹細胞」と呼ばれる万能細胞を用います。幹細胞は自分自身を複製できる能力(自己複製能)と体の様々な臓器・組織の細胞に変化できる能力(分化能)をもつ細胞のことを指します。 幹細胞治療では幹細胞のもつ分化能を利用しています。傷ついた部位に幹細胞を投与することでその臓器や器官が「再生する力」を引き出しているのです。 これまで、幹細胞治療は多くの疾患への可能性が示唆されてきましたが、神経もその一つです。従来、傷ついた神経組織は回復が非常に困難と考えられていました。しかし、最先端の再生医療では、この神経の修復もできるのではと期待されています。 https://www.youtube.com/watch?v=5HxbCexwwbE 日帰り再生医療の実際 当院では様々な神経損傷に対して幹細胞治療を提供しています。実際の治療の流れを3ステップでご説明します。 ①脂肪採取 診察や各種検査の結果から、幹細胞治療を行うことが決定したら、まずは幹細胞の採取を行います。当院では、患者様ご自身の脂肪から幹細胞を採取しています。お臍の下あたりから、脂肪を米粒2~3粒ほどの少量を採取します。傷は1cmほどと小さく、局所麻酔で可能な上、痛みもほとんどありません。 ②細胞培養・増殖 取り出した脂肪から、幹細胞を取り出して培養していきます。当院の細胞加工室の技術は国内トップクラスです。他施設で用いられる牛などの血清でなく、患者様ご本人の血清を用いた培養をすることで、感染症やアレルギーのリスクを抑えています。輸送や保存の際に幹細胞を凍結しないため、フレッシュで生き生きとした状態で細胞の投与が可能です。 ③患部への投与 4〜6週間かけて幹細胞を培養したら、患者様に投与を行います。当院では点滴だけでなく、「脊髄腔内ダイレクト注射療法」を行なっています。背中から細い針を刺して、幹細胞を脊髄のすぐ近くに直接注入する方法です。直に幹細胞を届けることができるため、神経の再生能力がより高まることが期待されます。投与は局所麻酔下で行い、当日にご帰宅いただけます。 まとめ・リゾトミーの術後後遺症にお悩みですか?日帰り最新医療のご紹介 リゾトミーと術後後遺症、また合併症治療の最先端についてご紹介いたしました。 つらい神経障害の後遺症に対してお困りであれば、幹細胞治療をご検討されてはいかがでしょうか。 当院ではしっかりご納得いただいた上で治療を選んでいただくことを大切にしております。お電話やメールでの無料相談も受け付けております。 気になることがあれば、まずはお気軽にご相談ください。 No.183 監修:医師 坂本貞範 参考文献 伊達久. 医学と薬学. 77(1): 31-37, 2020. 山口忍, 吉村文貴, 松本茂美, 竹中元康, 飯田宏樹. 日本臨床麻酔学会誌. 34(7): 938-946, 2014. 濱口眞輔, 山田哲平. 麻酔 68(9): 959-965, 2019. 奥田泰久. 日本臨床麻酔学会誌 38(5): 622-626, 2018. 金子和生, 田口敏彦. 日本腰痛学会雑誌 12(1):72-76, 2006. 腰痛診療ガイドライン2019 改訂第2版 Chen KT, Kim JS, Huang AP, Lin MH, Chen CM. Current Indications for Spinal Endoscopic Surgery and Potential for Future Expansion. Neurospine. 2023 Mar;20(1):33-42. doi: 10.14245/ns.2346190.095. Epub 2023 Mar 31. PMID: 37016852; PMCID: PMC10080449. 再生医療ポータル 再生医療について 再生医療の現状と展望
最終更新日:2024.04.10